Abstract

Background: The combination of the BCL2 inhibitor venetoclax with azacytidine (Ven+Aza) is efficacious for elderly patients with acute myeloid leukemia (AML) who are unfit for standard chemotherapy. However, the overall and progression-free survival of these patients after 1 year of therapy is approximately 30-40%, and resistance to Ven+Aza, either primary or adaptive through clonal evolution, limits its durability. Therefore, we evaluated new venetoclax combinations to elucidate mechanisms and biomarkers of response and resistance.
Methods: We tested a set of 31 venetoclax (Ven) combinations using an ex vivo assay on primary AML patient specimens (n=433, though not all combinations were screened for all samples). Enhanced efficacy was determined using a combination ratio (CR), defined as the sensitivity of the combination (by AUC) divided by the sensitivity of the most potent single agent AUC. Drug combination synergy was evaluated using the highest single agent (HSA) method. Clinical, genetic, and disease status information were collated, and ex vivo combination sensitivities were compared to matched single-agent data by Friedman test, across groups by Wilcoxon rank sum or Kruskal-Wallis test, and with continuous variables by Spearman rank correlation. For patient specimens with RNAseq data collected (n=279 patients), transcriptomic gene signatures of AML tumor cell state (van Galen, Cell 2019) were correlated with combination sensitivity by Spearman rank. Uni- and multi-variate analyses were applied to the full dataset to identify biomarkers of differential sensitivity to each combination. For one combination, Ven plus the JAK1/2 inhibitor ruxolitinib (Rux), additional genome wide CRISPR screens and follow up in vivo patient xenograft and mechanistic validation experiments using AML cell line models were performed.
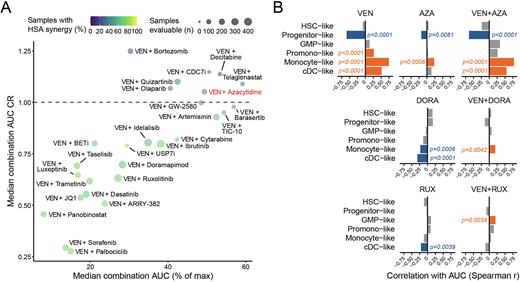
Results: Using Ven+Aza as a benchmark, we found several venetoclax combinations that exceeded both the ex vivo activity and enhanced efficacy observed with Ven+Aza in primary AML patient samples (Fig. 1A), while showing limited to no effect on stromal cell controls. Evaluation of clinical and genetic data with combination sensitivity revealed many significant transcriptomic, genetic, and immunophenotypic associations. The strong bias in sensitivity of Ven with respect to transcriptomic signatures of primitive tumor cell states compared to more mature, monocytic tumor states was unchanged with Ven+Aza and select other combinations. Importantly, this differential sensitivity was overcome when pairing Ven with several new drug partners such as Rux or the p38 MAPK inhibitor doramapimod (Fig. 1B). Uni- and multi-variate analyses further revealed an array of different biomarkers involving clinical and genetic features that correlated with significant differential sensitivity to each combination. Many of these likewise involved markers of cell differentiation state, such as CD14. To elucidate the mechanisms of enhanced efficacy of the Ven+Rux combination, which is currently under phase 1 clinical investigation, in vivo murine patient xenograft models and genome-wide CRISPR screens in OCI-AML2 cells were performed. Ven+Rux demonstrated superior inhibition of tumor growth in an AML xenograft model compared to either single-agent. The Ven+Rux CRISPR screen identified many genes whose knockdown was enriched in combination-resistant cells, including three (MDM2, MED13L, MEIS2) that were confirmed to confer resistance in subsequent immunoblotting and drug sensitivity validation experiments. In comparison, analysis of a control CRISPR screen using Ven alone revealed numerous gene hits in the JAK/STAT signaling pathway as enhancing the sensitivity of Ven.
Conclusion: To expand the utility of venetoclax-inclusive combinations, we prospectively profiled a set of drug combinations and identified several with activity that exceeds Ven+Aza in ex vivo assays. Associations of clinical features with sensitivities to these drug combinations provide insight into their effectiveness which may be used to align the most promising combinations with translation to treatment of discrete subsets of AML patients. Together, these findings support opportunities for expanding the impact of Ven-based drug combinations in AML by leveraging clinical and molecular biomarkers of response.
Disclosures
Nechiporuk:Engine Biosciences: Current Employment. Druker:US Patent: Patents & Royalties: 11049247; Sun Pharma Advanced Research Company: Patents & Royalties: Mutated ABL Kinase Domains (non-exclusive license); CytoImage: Patents & Royalties: QD Molecular Assay for Personalized Oncoprotein Detection in Leukemia (exclusive license); (Novartis exclusive license) and OHSU and Dana-Farber Cancer Institute (one Merck exclusive license, one CytoImage, Inc. exclusive license, and one Sun Pharma Advanced Research Company non-exclusive license): Patents & Royalties: Patent 6958335; US Patent: Patents & Royalties: 6958335; US Patent: Patents & Royalties: 4326534; Astra-Zeneca: Other: Clinical Trial Funding, Research Funding; Recludix Pharma: Other: Sponsored Research Agreement ; Enliven Therapeutics: Other: Sponsored Research Agreement ; VB Therapeutics: Other: Founder; Multicancer Early Detection Consortium: Membership on an entity's Board of Directors or advisory committees; Beat AML LLS: Other: Joint Steering Committee ; CureOne: Membership on an entity's Board of Directors or advisory committees; Burroughs Wellcome Fund: Membership on an entity's Board of Directors or advisory committees; Vincerx Pharma: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Amgen: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Recludix Pharma: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; GRAIL: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Iterion Therapeutics: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Enliven Therapeutics: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Blueprint Medicines: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Aptose Biosciences: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; RUNX1 Research Program: Other: Scientific Advisory Board ; Novartis: Other: Scientific Advisory Board; Clinical Trial Funding , Patents & Royalties: 6958335 (exclusive license), Research Funding; Nemucore Medical Innovations: Other: Scientific Advisory Board ; DNA SEQ: Other: Scientific Advisory Board ; Celgene: Other: Scientific Advisory Board ; Cepheid: Other: Scientific Advisory Board ; US Patent: Patents & Royalties: 7416873; US Patent: Patents & Royalties: 7592142; US Patent: Patents & Royalties: 10473667; Therapy Architects (ALLCRON): Other: Scientific Advisory Board ; Adela Bio: Other: Scientific Advisory Board ; Aileron Therapeutics: Other: Scientific Advisory Board ; Merck: Patents & Royalties: Monoclonal antiphosphotyrosine antibody 4G10; US Patent: Patents & Royalties: 10664967. Tyner:Astra-Zeneca: Research Funding; Constellation: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Incyte: Research Funding; Janssen: Research Funding; Kronos: Research Funding; Meryx: Research Funding; Petra: Research Funding; Schrodinger: Research Funding; Seattle Genetics: Research Funding; Syros: Research Funding; Takeda: Research Funding; Tolero: Research Funding; Recludix Pharma: Membership on an entity's Board of Directors or advisory committees; Array: Research Funding; Aptose: Research Funding; Agios: Research Funding; Acerta: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal